Section A: Sources and Fields
A1. Electric Charge
Sub Topics covered in this Module:
You have completed “Physics I”, a course dealing with mechanics and have now arrived at “Physics II”. In this course we will learn about electricity and magnetism – two topics that are fundamentally intertwined. In our approach we will drop the traditional sequence of studying electricity first, then magnetism, to finally discuss how they are connected. Instead, we take the bold stance to study electricity and magnetism side-by-side, in-unison from the get-go. This approach is in step with the present understanding of the subject: that electricity and magnetism are two manifestations of the same phenomenon that we call electromagnetism. Our approach allows us to compare, contrast and see the interwoven nature of electricity and magnetism at every step along the way providing much insight into the unified theory of electromagnetism – one of the most profound and beautiful theories in Physics.
What makes this unified theory of electromagnetism so profound is that it explains with exceptional precision a fundamental interaction of nature. An interaction so fundamental that it shapes the universe, unconditioned by time – from the big bang to the present to the future.
What is also remarkable is that electromagnetic theory, as we know it today, cannot be attributed to the work of any one scientist alone. It is the contribution of many brilliant scientists, each building a little on what was known at their time, that culminated in this unified theory – a theory that was well developed by the end of the 19th century. But understand that electromagnetism existed in all its glory before civilization happened. With the insights of these brilliant scientists, man figured out how to manipulate it, understand it and put it to use. So much so that it is now integral to our lifestyle.

To provide a description of our world that includes electricity, magnetism, and electric and magnetic interactions, we will introduce at the outset a new concept – a big idea that we call a “field”. What is a field? To answer this, it would help if we first asked what creates a field? Perhaps in your Physics I class you learnt about the gravitational field. What created this gravitational field that was central to most of the mechanics you studied in Physics I? What was its source?
Concept check
Question A1
If you answered the above question, you might already be thinking about what is common for all these objects. Did you stumble upon mass? Mass is a property that all these objects have in common. The source of the gravitational field is mass. But what is mass?
Give your best definition for mass. Avoid using words like matter and material in your definition. You’ve got this!
If you tried to look it up, you may have realized that “mass” was never defined for you in Physics I. Yet, it was used throughout. Let us take an example from Physics I where you shoot a basketball. You know the basketball goes up and then comes back down. Why does it come down? Because it has mass. But you never asked what mass is (just like you never asked what a basketball is because you intuitively know what it is). In the case of mass, you think you intuitively know what mass is. In fact, your intuitive experience is the weight of the basketball not its mass. The weight is the interaction between the “mass” of the basketball and the gravitational field that it is in (in this case the gravitational field of the earth). The earth has mass which generates its gravitational field. The basketball also has mass and therefore it too has a gravitational field around it (of course, much weaker). It turns out that we cannot truly define mass. It is a fundamental property – a property that generates a gravitational field.
So, what creates an electric field? And what creates a magnetic field? Electric charge is a source that generates an electric field. And the motion of an electric charge is a source that generates a magnetic field.
Give your best definition for electric charge. Think about the previous discussion for mass…
Electric charge is also a fundamental property just like mass is a fundamental property. The best you can do to define charge is to say it is a source that generates an electric field.
Electric charge comes in two flavors. Conventionally we call them positive charge and negative charge. Some elementary and sub-atomic particles are endowed with charge. Like electrons, protons, muons, and tau particles (see table A1). Others are not, like neutrons and neutrinos – we call them charge neutral.
SI unit for charge: Coulomb (C)
|
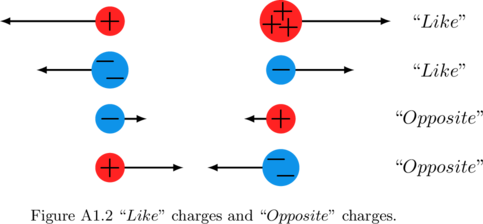
Let us consider two charges. They could both be positive, or both be negative. In this case, when they are both of the same sign, we call them “like” charges. “Like” charges repel each other. Note, they do not have to be of equal magnitude to be called “like” charges, they just have to be of the same sign. On the other hand, the two charges could be of opposite signs: one could be positive and the other negative. In this case we call them “opposite” charges. “Opposite” charges attract. |
|
Concept check
Question A2
Concept check
Question A3 and A4
Concept check
Question A5
Let us discuss some properties of electric charge.
- Electric charge is quantized.
- Electric charge is conserved.
Charge Quantization
A selected set of particles are listed in Table A1 together with their charge and their mass. From a glance at the table, you will notice that an electron is said to have a negative charge and a proton a positive charge, but this is only by convention, and we will stick by this convention. You will also notice that an electron has the same amount of charge as a proton. It turns out that this is the smallest unit of charge that can be isolated. This amount of charge, which has been precisely measured, is called the fundamental unit of charge (or sometimes called the elementary charge) and is denoted “![]() ”.
”.
Elementary unit of charge
![]()
This is a fundamental physical constant of nature.
Notice that the charge of each particle given in Table A1 is a multiple of ![]() . This applies to macroscopic objects as well. Thus, electric charge is quantized in units of
. This applies to macroscopic objects as well. Thus, electric charge is quantized in units of ![]() .
.
The charge of any object, ![]() can be given by
can be given by
![]()
where ![]() is a positive integer and
is a positive integer and ![]() depends on the sign of the charge.
depends on the sign of the charge.
In calculations, sometimes it is convenient to work in units of ![]() (the fundamental unit of charge) since the SI unit (1 Coulomb) is a large amount of charge.
(the fundamental unit of charge) since the SI unit (1 Coulomb) is a large amount of charge.
Note: While we have a smallest unit of charge that can be isolated, there is no such smallest unit for mass. Looking at table A1, you will notice that all the different particles have different masses. Unlike charge, mass is not quantized.
Charge can move from one entity to another, but it cannot be created or destroyed. Thus, electric charge is conserved: in a closed system, the total amount of charge always stays the same. For example, any chemical reaction or nuclear reaction is consistent with the law of conservation of charge.
Chemical reaction:
![]()
![]() charge is conserved
charge is conserved
Nuclear reaction:
![]()
![]() charge is conserved
charge is conserved
(In ![]() nucleus, 92 represents the atomic number. It is the number of protons in the nucleus. 238 represents the mass number. It is the total number of protons and neutrons in the nucleus.)
nucleus, 92 represents the atomic number. It is the number of protons in the nucleus. 238 represents the mass number. It is the total number of protons and neutrons in the nucleus.)
Concept check
Question A6
![]()
Conductors and insulators are the names given to materials based on their ability to allow electric charge to move through them. Conductors allow charge to move through them easily; we say that they conduct electricity. Whereas insulators are materials that do not conduct electricity well; they do not allow electric charge to easily move through them.
Metals like copper and gold are examples of good conductors. In general, all metals have what are called conduction electrons (also referred to as “free” electrons). These conduction electrons are weakly bound to the atoms and hence are “free” to move around the metal making them conductors.
An insulator is a material in which the electrons are tightly bound to the atoms and hence cannot move around easily. Take quartz for example; it is made of silicon and oxygen atoms where the electrons are tightly bound to these atoms and therefore are not free to move, making quartz an insulator.
One of man’s first controlled encounters with electric charge was in the process of rubbing together two objects (as opposed to uncontrolled encounters such as lightning bolts). In ancient times people observed a mysterious phenomenon when, for example, a piece of amber was rubbed on animal fur: the amber was found to attract small pieces of dust even without direct contact (see the discussion on “polarizing an insulator“).
The Greek word for amber is “elektron”. This is where we get the words “electron” and “electricity”.
It turns out that the act of rubbing transfers some electrons from the animal fur onto the piece of amber. Based on our convention, this leaves the piece of amber negatively charged. This also leaves the animal fur positively charged because we removed some negatively charged electrons from it. Note that this process is consistent with charge conservation discussed above: For example, if the piece of amber gained 337 electrons or a charge of ![]() , then the animal fur lost exactly 337 electrons giving it a charge of
, then the animal fur lost exactly 337 electrons giving it a charge of ![]() .
.
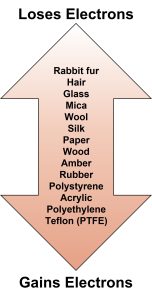
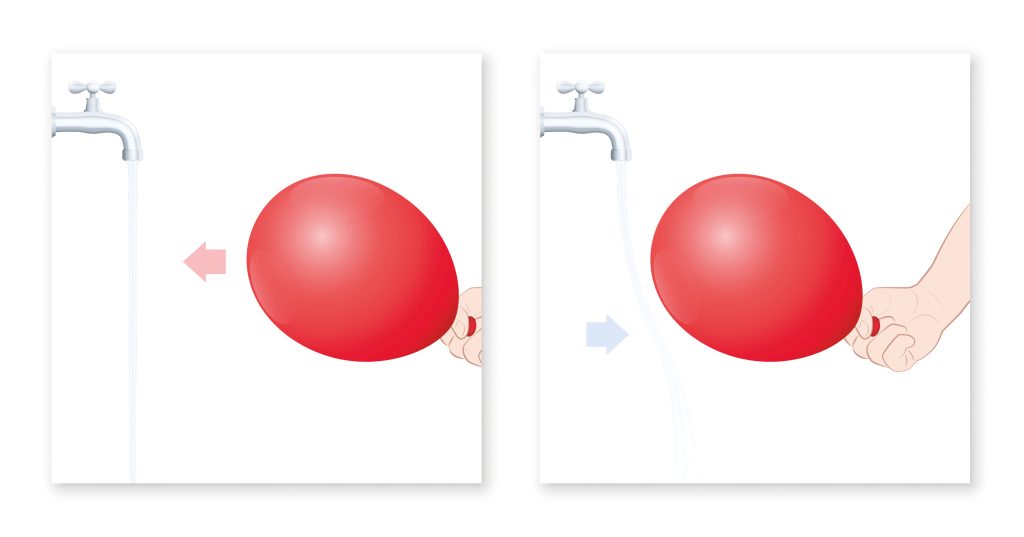
In modern times we can make similar observations by rubbing a piece of plastic on a piece of silk cloth or rubbing a rubber balloon on our hair. In each case, the act of rubbing transfers electrons from one material to another making one of the materials negatively charged and the other positively charged. Which material loses electrons and which material acquires these electrons can be empirically determined and depends on how strong these electrons are bonded to the atoms in the material (see Figure A1.3: Triboelectric series).
Images below show examples of charged objects where static electricity is observed.
 |
 |
 |
Concept check
Question A7
Macroscopic objects, made of atoms, are neutral most of the time. As we know, atoms have protons and neutrons in the nucleus and electrons moving around the nucleus. Each atom has the same number of protons (+ charges) and electrons (- charges) which gives them a net charge of zero and hence charge neutral. In fact, it is the attraction between opposite charges (protons and electrons) that makes atoms stable.
We can make a macroscopic object charged by stripping some electrons off it (making it positively charged) or by adding some electrons to it (making it negatively charged). The net charge of the object, ![]() can be given by
can be given by
![]()
Where ![]() is the total number of protons and
is the total number of protons and ![]() is the total number of electrons.
is the total number of electrons. ![]() is always an integer: consistent with charge being quantized. The charge of the object would be an integer multiple of
is always an integer: consistent with charge being quantized. The charge of the object would be an integer multiple of ![]() .
.
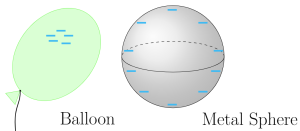
If the macroscopic object that was charged is an insulator (Figure A1.7, balloon), this extra charge stays localized (i.e. stays where it was placed). In contrast, if charge is placed on a conductor such as a metal object (Figure A1.7, metal sphere), the conduction electrons in the metal will redistribute such that the extra charge is spread out across the conductor to be far away from each other, consistent with the fact that like charges repel.
Note: Eventually, after a long time, ions in the atmosphere will contact the material and neutralize the charge.
Polarization is a phenomenon where the charge distribution of an object is skewed such that one side of the object is more positively charged and the other side of the object is more negatively charged. While the object as a whole is neutral, the separation of the charge makes it polarized. This can be achieved without physical contact with the object.
Polarizing a metal: A charged balloon polarizes a conducting (metal) tumbler. See the video clip below and answer Question A8. The balloon never touches the tumbler, but the metal tumbler is polarized since its conduction electrons are repelled by the negatively charged balloon. Note that conduction electrons (or “free” electrons) cause a charge separation over macroscopic distances when a metal is polarized.
Polarization: Rolling a Tumblr
Concept check
Question A8

A conductor can also be charged by induction. It is a two-step process:
- Polarize the conductor.
- Keeping the conductor polarized, remove the separated charge from one side.
Step 2 generally involves connecting the conductor with a wire to the ground – known as “grounding”. The earth can be thought of as an enormous reservoir of electric charge. We call it an “electrical ground”. By connecting the polarized conductor to the earth, the charge from one side of the conductor will selectively move through the wire into the electrical ground. Once the wire is removed the conductor is left charged. Consider the example below.
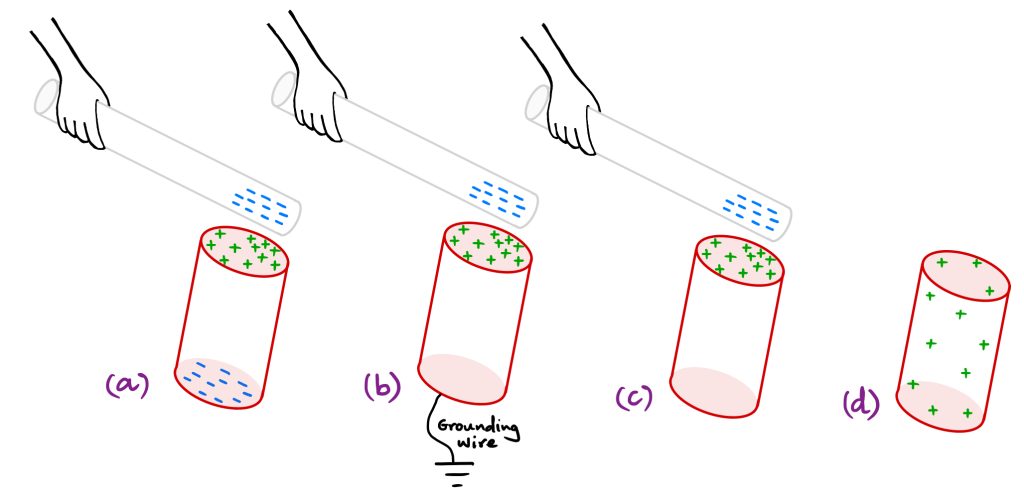
A negatively charged rod (say a Teflon rod rubbed with a silk cloth) is brought close to the top surface of an empty aluminum soda-can. See Figure A1.9. The free electrons in the aluminum metal are repelled by this negatively charged rod polarizing the soda-can: the top surface of the can positive, and the bottom surface negative. Keeping the negatively charged rod close to the top surface so that the soda-can stays polarized, a wire is connected from the bottom of the can to the ground. The excess electrons that were repelled by the negatively charged rod to the bottom of the can are now pushed through the grounding wire into the electrical ground making the bottom of the can charge neutral. The top of the can remains positively charged due to the attraction with the negatively charged rod. Once the grounding wire is removed, we have a soda-can with excess positive charge. If the negatively charged rod is now moved away, the excess positive charge will distribute over the surface of the soda-can. The soda-can has been charged by induction.
An intelligent being in a distant galaxy could pick a convention where the signs are flipped (or pick an entirely different convention like electrons are “blue” and protons are “red” to distinguish between the two flavors of charge) and independently develop an equally valid and accurate theory consistent with the convention they picked.
That electrons have a negative charge