22 4.5 Catabolism of Lipids and Proteins
Learning Objectives
- Describe how lipids are catabolized
- Describe how lipid catabolism can be used to identify microbes
- Describe how proteins are catabolized
- Describe how protein catabolism can be used to identify bacteria
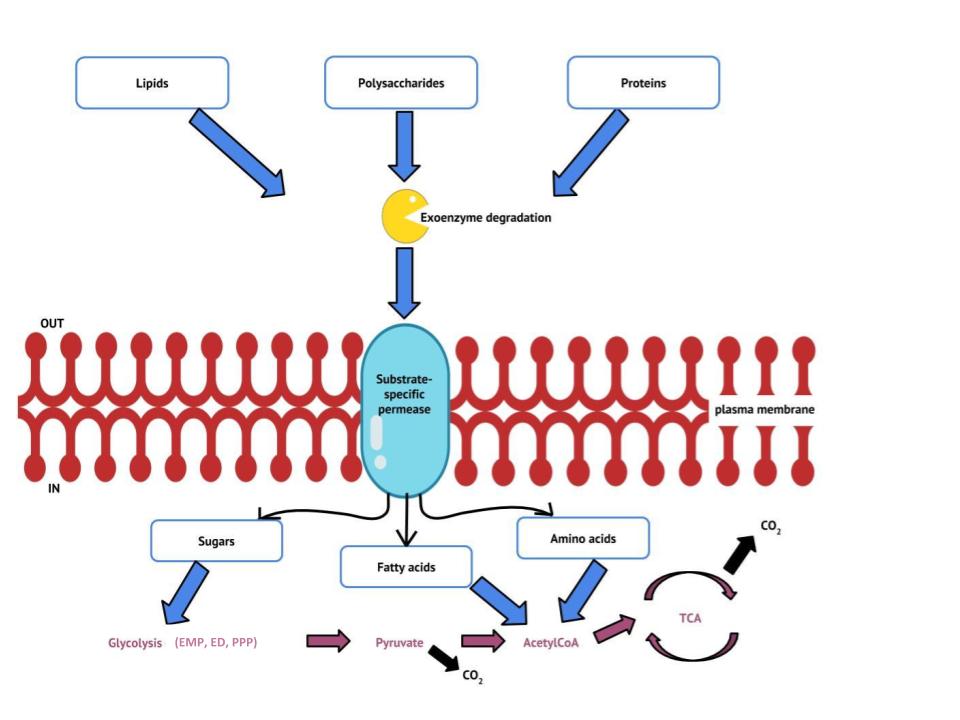
Previous sections have discussed the catabolism of glucose, which provides energy to living cells, as well as how polysaccharides like glycogen, starch, and cellulose are degraded to glucose monomers. But microbes consume more than just carbohydrates for food. In fact, the microbial world is known for its ability to degrade a wide range of molecules for use as carbon sources, both naturally occurring and xenobiotic: those made by human processes. In this section, we will see that the pathways for both lipid and protein catabolism connect to those used for carbohydrate catabolism, eventually leading into the central pathways: glycolysis, the transition reaction, the pentose phosphate pathway and the Krebs cycle (Figure 4.25). Metabolic pathways should be considered to be porous—that is, substances enter from other pathways, and intermediates leave for other pathways. These pathways are not closed systems. Many of the substrates, intermediates, and products in a particular pathway are reactants in other pathways. The figure below summarizes the flow of major organic energy sources into these central pathways.
CLINICAL FOCUS: Part 3
Because bacterial meningitis progresses so rapidly, Hannah’s doctors had decided to treat her aggressively with antibiotics, based on empirical observation of her symptoms. However, laboratory testing to confirm the cause of Hannah’s meningitis was still important for several reasons. N. meningitidis is an infectious pathogen that can be spread from person to person through close contact; therefore, if tests confirm N. meningitidis as the cause of Hannah’s symptoms, Hannah’s parents and others who came into close contact with her might need to be vaccinated or receive prophylactic antibiotics to lower their risk of contracting the disease. On the other hand, if it turns out that N. meningitidis is not the cause, Hannah’s doctors might need to change her treatment.
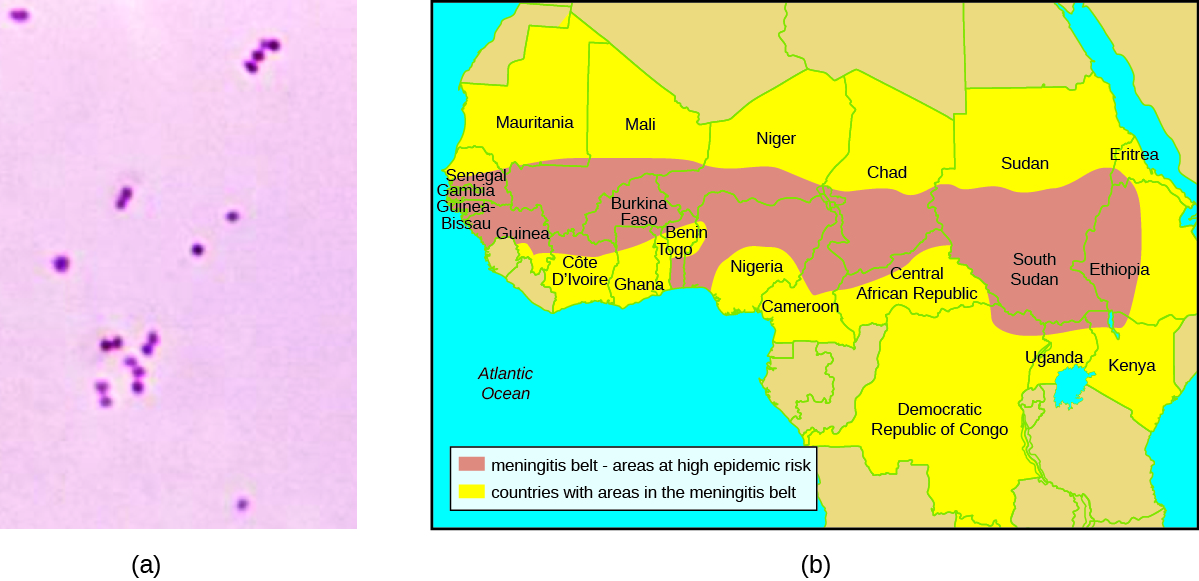
The clinical laboratory performed a Gram stain on Hannah’s blood and CSF samples. The Gram stain showed the presence of a bean-shaped gram-negative diplococcus. The technician in the hospital lab cultured Hannah’s blood sample on both blood agar and chocolate agar, and the bacterium that grew on both media formed grey, non-haemolytic colonies. Next, he performed an oxidase test on this bacterium and determined that it was oxidase positive. Last, he examined the repertoire of sugars that the bacterium could use as a carbon source and found that the bacterium was positive for glucose and maltose use but negative for lactose and sucrose use. All of these test results are consistent with characteristics of N. meningitidis.
- What do these test results tell us about the metabolic pathways of N. meningitidis?
- Why do you think that the hospital used these biochemical tests for identification in lieu of molecular analysis by DNA testing?
Go to next Clinical Focus box. Go back to the previous Clinical Focus box.
Lipid Catabolism
Triglycerides are a form of long-term energy storage in animals. They are made of glycerol and three fatty acids (see Lipids). Phospholipids compose the cell and organelle membranes of all organisms except the archaea. Phospholipid structure is similar to triglycerides except that one of the fatty acids is replaced by a phosphorylated head group ( Lipids). Triglycerides and phospholipids are broken down first by releasing fatty acid chains (and/or the phosphorylated head group, in the case of phospholipids) from the three-carbon glycerol backbone. The reactions breaking down triglycerides are catalyzed by lipases and those involving phospholipids are catalyzed by phospholipases. These enzymes contribute to the virulence of certain microbes, such as the bacterium Staphylococcus aureus and the fungus Cryptococcus neoformans. These microbes use phospholipases to destroy lipids and phospholipids in host cells and then use the catabolic products for energy (see Virulence Factors of Bacterial and Viral Pathogens).
The resulting products of lipid catabolism, glycerol and fatty acids, can be further degraded. Glycerol can be phosphorylated to glycerol-3-phosphate and easily converted to glyceraldehyde 3-phosphate, which continues through glycolysis. The released fatty acids are catabolized in a process called β-oxidation, which sequentially removes two-carbon acetyl groups from the ends of fatty acid chains, reducing NAD+ and FAD to produce NADH and FADH2, respectively, whose electrons can be used to make ATP by oxidative phosphorylation. The acetyl groups produced during β-oxidation are carried by coenzyme A to the Krebs cycle, and their movement through this cycle results in their degradation to CO2, producing ATP by substrate-level phosphorylation and additional NADH and FADH2 molecules (see Appendix C – Metabolic Pathways for a detailed illustration of β-oxidation).
Other types of lipids can also be degraded by certain microbes. For example, the ability of certain pathogens, like Mycobacterium tuberculosis, to degrade cholesterol contributes to their virulence. The side chains of cholesterol can be easily removed enzymatically, but degradation of the remaining fused rings is more problematic. The four fused rings are sequentially broken in a multistep process facilitated by specific enzymes, and the resulting products, including pyruvate, can be further catabolized in the Krebs cycle.
- How can lipases and phospholipases contribute to virulence in microbes?
Protein Catabolism
Proteins are degraded through the concerted action of a variety of microbial protease enzymes. Extracellular proteases cut proteins internally at specific amino acid sequences, breaking them down into smaller peptides that can then be taken up by cells. Some clinically important pathogens can be identified by their ability to produce a specific type of extracellular protease. For example, the production of the extracellular protease gelatinase by members of the genera Proteus and Serratia can be used to distinguish them from other gram-negative enteric bacteria. Following inoculation and growth of microbes in gelatin broth, degradation of the gelatin protein due to gelatinase production prevents solidification of gelatin when refrigerated. Other pathogens can be distinguished by their ability to degrade casein, the main protein found in milk. When grown on skim milk agar, production of the extracellular protease caseinase causes degradation of casein, which appears as a zone of clearing around the microbial growth. Caseinase production by the opportunist pathogen Pseudomonas aeruginosa can be used to distinguish it from other related gram-negative bacteria.
After extracellular protease degradation and uptake of peptides in the cell, the peptides can then be broken down further into individual amino acids by additional intracellular proteases, and each amino acid can be enzymatically deaminated to remove the amino group. The remaining molecules can then enter the transition reaction or the Krebs cycle.
- How can protein catabolism help identify microbes?
CLINICAL FOCUS: Resolution
Although there is a DNA test specific for Neisseria meningitidis, it is not practical for use in some developing countries because it requires expensive equipment and a high level of expertise to perform. The hospital in Banjul was not equipped to perform DNA testing. Biochemical testing, however, is much less expensive and is still effective for microbial identification.
Fortunately for Hannah, her symptoms began to resolve with antibiotic therapy. Patients who survive bacterial meningitis often suffer from long-term complications such as brain damage, hearing loss, and seizures, but after several weeks of recovery, Hannah did not seem to be exhibiting any long-term effects and her behaviour returned to normal. Because of her age, her parents were advised to monitor her closely for any signs of developmental issues and have her regularly evaluated by her pediatrician.
N. meningitidis is found in the normal respiratory microbiota in 10%–20% of the human population.[1] In most cases, it does not cause disease, but for reasons not fully understood, the bacterium can sometimes invade the bloodstream and cause infections in other areas of the body, including the brain. The disease is more common in infants and children, like Hannah.
The prevalence of meningitis caused by N. meningitidis is particularly high in the so-called meningitis belt, a region of sub-Saharan African that includes 26 countries stretching from Senegal to Ethiopia (Figure 4.26). The reasons for this high prevalence are not clear, but several factors may contribute to higher rates of transmission, such as the dry, dusty climate; overcrowding and low standards of living; and the relatively low immunocompetence and nutritional status of the population.[2] A vaccine against four bacterial strains of N. meningitidis is available. Vaccination is recommended for 11- and 12-year-old children, with a booster at age 16 years. Vaccination is also recommended for young people who live in close quarters with others (e.g., college dormitories, military barracks), where the disease is more easily transmitted. Travellers visiting the “meningitis belt” should also be vaccinated, especially during the dry season (December through June) when the prevalence is highest.[3][4]
Go back to the previous Clinical Focus box.
Key Takeaways
- Collectively, microbes have the ability to degrade a wide variety of carbon sources besides carbohydrates, including lipids and proteins. The catabolic pathways for all of these molecules eventually connect into glycolysis and the Krebs cycle.
- Several types of lipids can be degraded by microbes. Triglycerides are degraded by extracellular lipases, releasing fatty acids from the glycerol backbone. Phospholipids are degraded by phospholipases, releasing fatty acids and the phosphorylated head group from the glycerol backbone. Lipases and phospholipases act as virulence factors for certain pathogenic microbes.
- Fatty acids can be further degraded inside the cell through β-oxidation, which sequentially removes two-carbon acetyl groups from the ends of fatty acid chains.
- Protein degradation involves extracellular proteases that degrade large proteins into smaller peptides. Detection of the extracellular proteases gelatinase and caseinase can be used to differentiate clinically relevant bacteria.
Multiple Choice
Fill in the Blank
Short Answer
- How are the products of lipid and protein degradation connected to glucose metabolism pathways?
- What is the general strategy used by microbes for the degradation of macromolecules?
Critical Thinking
- Do you think that β-oxidation can occur in an organism incapable of cellular respiration? Why or why not?
- Centers for Disease Control and Prevention. “Meningococcal Disease: Causes and Transmission.” http://www.cdc.gov/meningococcal/about/causes-transmission.html. ↵
- Centers for Disease Control and Prevention. “Meningococcal Disease in Other Countries.” http://www.cdc.gov/meningococcal/global.html ↵
- Centers for Disease Control and Prevention. “Health Information for Travelers to the Gambia: Traveler View.” http://wwwnc.cdc.gov/travel/destinations/traveler/none/the-gambia ↵
- Centers for Disease Control and Prevention. “Meningococcal Disease: Vaccination.” http://www.cdc.gov/vaccines/vpd-vac/mening/who-vaccinate.htm ↵
