5 Micro and Biofluidic Devices
5.0 Learning Objectives
- Analyse macro and micro- and nano-fabricated devices in the context of theory learnt
- Discussion of leading industry examples
- Introduce concepts to developing and designing a simple microfluidics device
5.1 Macro-fabricated devices – Macro-mixer
5.1.1 Mechanical heart therapy
Macrofluidics can analyse and support in haemodynamics and large cardiovascular system components. Given the scarcity of heart transplants, blood pumps are the most effective and efficient solution to heart failure. Maintaining or supporting systemic circulation requires 3-7 L/min of blood, a relatively high amount of flow. Given extensive wear and tear of pulsatile components and the high rate of malfunction in pulsatile pumps, the most widespread pumps are rotary blood pumps.
The pumping of blood through nonphysiological geometries and materials has unique complications such as hemolysis, thromboembolitic events and pump thrombosis. These events are counteracted by three actions guiding pump design: [15]
- Reduce high shear regions in the pump
- Reduce the exposure time of blood to high shear regions
- Reduce the percentage of blood that is exposed to high shear regions
5.1.2 Macro-mixer
Mixers can be used to mix fluid. These devices widely applied in following areas like:
- Manipulate sample concentration
- Chemical synthesis and reactions (protein folding)
- Extraction
- Purification
- Biological analysis
- Droplet/ emulsion process
The macro-mixer shown in Figure 5.1(a) is a device to illustrate macro-fabrication and the mixing of two fluids at macroscopic scale, with Reynolds numbers usually bigger than one. Macro-scale channels can be created on a mould. 3D printing is a very efficient technique for fabrication of macro-mixers. After 3D printed macro-scale channel mould, de-mould PDMS macro-channel by pouring PDMS in the mould and let it settle. Then the PDMS chip with punched inlet/outlet holes can bond with a glass/PDMS substrate and put into future application.
The mixing of fluids often relies on convection effects
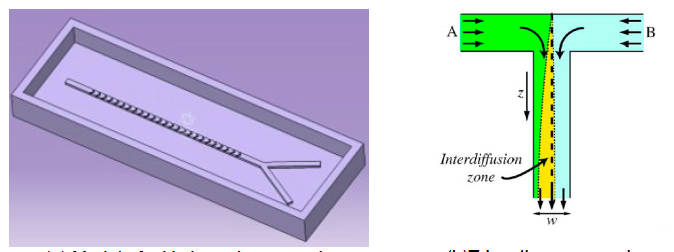
Figure 5.1 Different types of macro-mixer: a) Y-junction, b) T-junction
As shown in Figure 5.1(b), two fluids are injected and flow alongside each other in-side of the T-junction macromixer. For high Reynolds number fluids, eddies chaotically stretch and fold fluid elements. Turbulent mixing and thermal convection subsequently move fluids. For low Reynolds number fluids, mixing occurs by diffusion only in the interdiffusion zone. However, diffusion is a remarkably slow process which leads to long mixing time.
The macro-mixer shown has a low efficiency, because it requires a sufficient length for high quality mixing. Another problem associated with macro-mixers is that they can’t be added to microfluidic chips (a set of microfluidic channels etched or moulded into a material) due to their larger size. Additionally, macro-mixers experience inefficient mixing, due to low surface to volume ratio and the low heat and mass transfer efficiency.
5.1.3 Macro-stent
The narrowing of vessels in the cardiovascular system is a crucial components in most diseases, most commonly artherosclerosis. A stent is a surgically introduced tubular device that applies radial pressure in the vessel to ensure proper vessel diameter, however this often results in in-stent restenosis. This has lead to a new generation of drug eluting stents, which use biocompatible polymer coating over a metallic back-bone to continuously release medication. to reduce the risk of restenosis and thrombosis. The current techniques that are used in stent fabrication are etching, micro-electro discharge machining, electroforming, die-casting, and laser cutting.[16]
5.1.4 Replacement heart valves
Mechanical valves are fabricated from biocompatible materials intended to last through the entirety of the patients’ life. However, non-physiological geometries and materials will promote thrombosis. Therefore, patients need to undergo a chronic use of blood thinner medication to avoid thrombosis.
Bioprosthetic valves are created from animal donor valves or through the use of animal tissue with favourable mechanical properties. They do not require the chronic use of blood thinners, however this type of valve is often subject to calcification and a higher deterioration rate.[17]
5.2 Micro-fabricated devices and applications
Common microfluidic devices include external flow control, microvalves, micro-pumps, microflow sensors, microneedles, micromixers, microdispensers, microfilters and separators and microreactors.
Common devices for biofluids and cell study are microfluidic channels and chambers made from silicon, glass or plastic. Most commercial microfluidic devices are developed in plastic (considering its cheaper price). Applications for microfluidic channels regarding biofluids and cell study include mixing and homogenisation of different fluids, cell separation, cardiac-like flow generators and mimicry of blood microcirculation in microchannels.
Methods for microfluidic channel fabrication are either through moulding or direct. The moulding method uses photolithography, which will be explained in the next chapter. Additive manufacturing with high printing resolution (FDM, SLA, etc.) as well as CNC machining can fabricate precise moulds for moulding methods. Moulding through photolithography can be summarised into following steps:
- Cleaning
- Spin coating
- UV exposure
- Developing
- Inspection and hard baking
- Moulding
- Bonding to substrate
Direct fabrication mainly refers to additive manufacturing (SLA, 2-photon polymerisation (2PP) etc.). Microfluidic devices may also be 3D printed through stereolithography (SLA) in order to directly make microfluidic channels. Key advantages of direct additive manufacturing over moulding include its great material range and properties such as biocompatibility, transparency, elasticity, and gas permeability.
The required sample volume for different applications in biofluidics and cell study are listed in Figure 5.2.
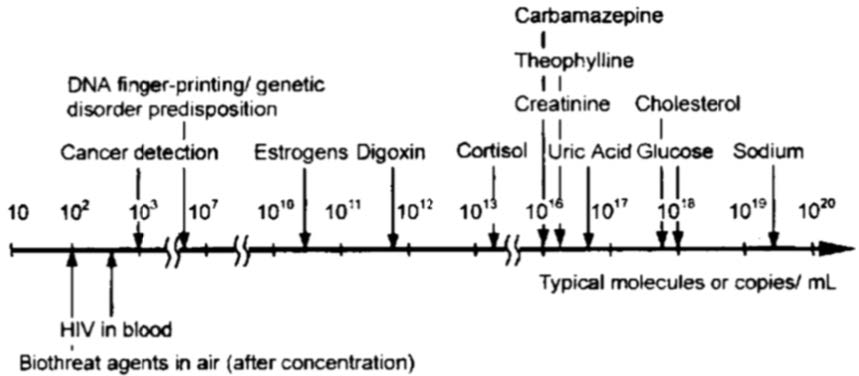
Figure 5.2 Required sample volume for different tests
Following agent can help to detect of biomolecules in micro-scaled channel.
- Digoxin – heart stimulating drug
- Cortisol – stress hormone from adrenal gland
- Creatinine – level in blood is measure of kidney function
- Theophylline – drug used to treat respiratory diseases, e.g. asthma
Some examples of biofluids and cell study in micron-scale will be discussed in the following.
5.2.1.1 Cell separation
Cell separation, which separates blood cells in a spiral microfluidic device, is one novel example of biofluids and cell study in micron-scale. This method utilises effects of curvature on the focusing positions inside of the micro-channels. Larger particles focus in a single position closer to the inner channel wall due to Net Lift Force while smaller particles under Dean Force will more easily to focus to the centre of channel. This phenomenon is exhibited in Figure 5.3. An appropriate outlet system can enhance the collection of the particles/cells sorted according to their sizes.
In the real life applications such as Point-of-Care Cancer Diagnostic and Treatment, cell separation has 100% sensitivity in diagnose. Its silicon based organic polymer is bio-compatibility and economic feasibility.
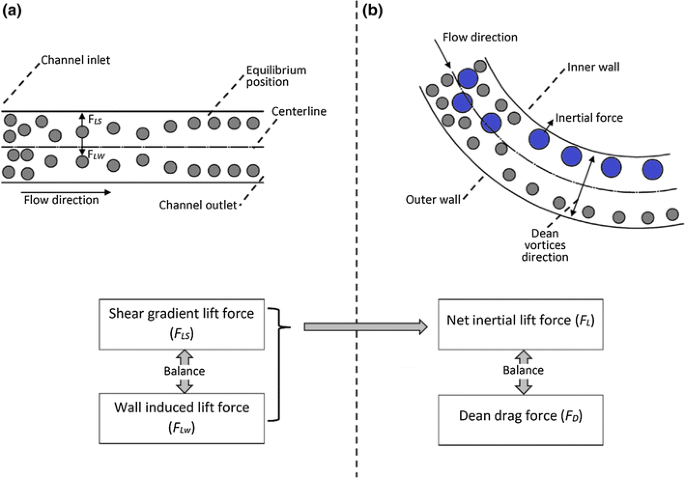
5.2.1.2 Mixing and homogenisation
Micro-scale mixers can fit into a micron size chip, unlike their macro-sized counterparts. It has a larger surface-to-volume ratio, which significantly increases mixing efficiency. Furthermore, it increases heat and mass transfer efficiency through the incorporation of complex 3D microstructures. The fluid type inside of the channel is laminar flow, with the Reynolds number less than 1. A few types of micro-mixers can be seen in Figure 5.4.
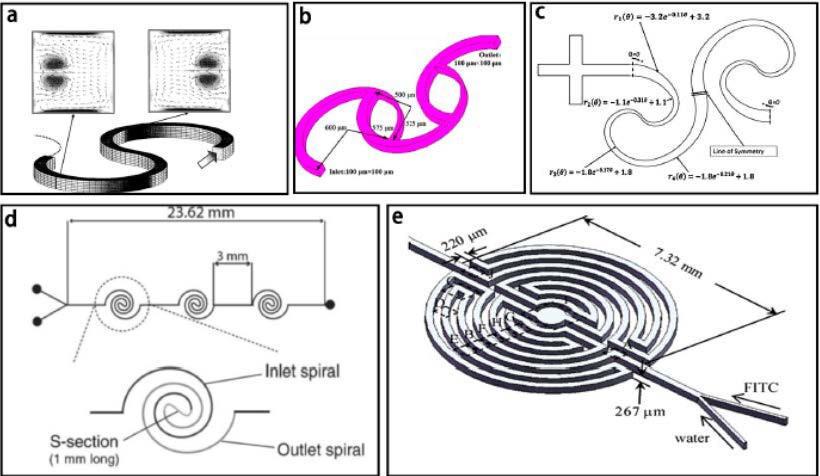
Micromixers can be active or passive. Active mixers may use capillary forces, are pressure driven, centrifugal, use inertia forces, light driven, use acoustics or electrokinetics. Passive micromixers utilize no energy input except the mechanism (pressure head) used to drive the fluid flow at a constant rate. Due to the dominating laminar flow on the microscale, mixing in passive micromixers relies mainly on chaotic advection realized by manipulating the laminar flow in microchannels or molecular diffusion with increasing the contact surface and time between the different fluid flows.[14]
5.2.2 Drug delivery
Shrinking a needle to micron dimensions makes use of its powerful delivery capabilities, while improving patient compliance and safety. In addition, a microneedle allows for precise tissue localisation of delivery, such as within the skin, the suprachoroidal space of the eye, and the cell nucleus. A microneedle should be large enough to deliver almost any drug or small particulate formulation while still being small enough to avoid pain, fear and the need for expert training to administer. Microneedles are usually fabricated sing photolithography and are made of polydimethylsiloxane (PDMS). The working principle of micro-needle can be seen in Figure 5.5.
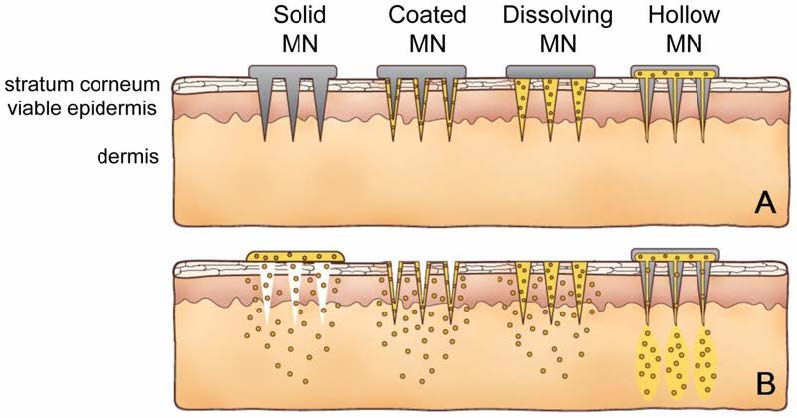
Micro-needles are first applied to the skin (A) and then used for drug delivery (B).
5.2.3 Microfluidics printers
5.2.3.1 Thermal jet printing
Printers that use the thermal jet printing method are equipped with a heated ink-filled chamber. By heating the liquid ink, an ink-bubble forms in the chamber, which then drops onto the paper as a tiny droplet. After that, the chamber is refilled with ink through microscopic channels and the process starts over.
5.2.3.2 Piezoelectric printing
Piezoelectric printers use piezoelectric crystals to act as a pump to force the ink into the nozzle tip. The piezoelectric crystal expands or contracts to move the fluid from the reservoir through the nozzle where it then hits the paper.
5.2.3.3 Bioprinting
Bioprinting uses printing actuators like piezoelectric crystals, inkjet nozzle, acoustic actuators or a laser print head to eject biomaterial out of the reservoir. Biomaterials such as cells and growth factors are combined to create tissue-like structures that imitate natural tissues. Living cell suspension is utilised instead of a thermoplastic or a resin – forming novel material known as bioink. Recently, technology advancements has even allowed for production of cartilage tissue.
5.2.4 Bioresorbable material for artery diseases
The use of bioresorbable stents in interventional cardiology presents a novel approach in the treatment of coronary artery disease. Stents are tiny wire mesh tubes which help keep coronary arteries open and reduce the chance of a heart attacks. Once implemented, stents are left in the artery permanently. Stents may be fabricated with a method called wet-spinning, incorporating either polymers (e.g. Poly-L-Lactic Acid (PLLA)) or metals (e.g. stainless steel or cobalt chrome alloys). To decide which material is used, properties like biocompatibility and biodegradability, as well as mechanical properties and physio-chemical properties must be considered.
5.2.3.4 Bioresorbable stent
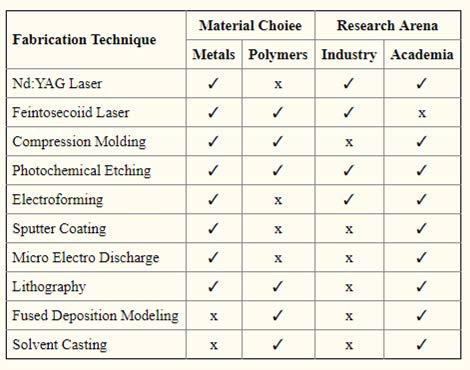
Bioresorbable stents are a technology used in interventional cardiology for the treatment of coronary artery diseases (CAD). The fabrication strategies can be seen in Table 5.1.
5.3 Nano-fabricated devices and systems
5.3.1 Transport phenomenon in nanochannels
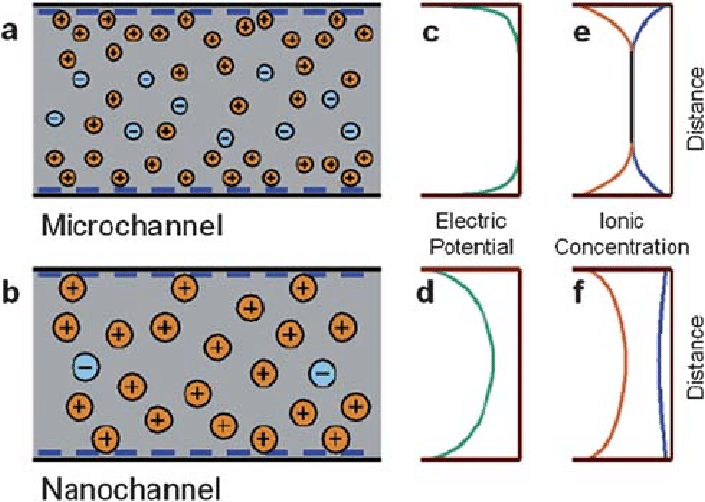
Specificities of liquid flow at the microscale such as the laminar flow or the high surface-to-volume ratio makes electro-kinetic, capillary or heat transfer effects particularly different when compared to nanoscale flow. The electro-kinetic transport phenomenon describes the phenomenon where surface charge density of fluid flow is not neutral in nanochannels (unlike in microchannels). Because of the electroneutrality requirement, the ratio of counterions to coions in the channel increases substantially and the electric potential isn’t neutral anymore. For instance, the charge density could change so that molecules/particles with counterion charges will get a higher transport rate. This is seen in Figure 5.6 below.
5.3.2 Pressure drop in a nanochannel
Transport processes in channels strongly depend on the surface-to-volume ratio of the channel. The smaller the channel gets, the higher the surface-to-volume ratio is; this leads to unique transport processes in nanochannels. Forces resulting from pressure, inertia, viscosity or gravity, which usually play a dominant role become more irrelevant on such a reduced scale. Instead, forces like pressure drop, surface tension and the applied potential become dominant in nanochannels. When the channel height is reduced, but the flowrate is supposed to be constant, the pressure drop and the voltage required increases (Figure 6.3). The required pressure drop increases substantially faster than the required voltage, making it easier to transport fluids in nanoscale systems using electro-kinetic flow.
![5.7 Figure 5.5 Required pressure and voltage drop for nanochannels with different channel heights [9]](https://oer.pressbooks.pub/app/uploads/sites/28/2020/11/pressurechannel.jpg)
5.3.3 Nanocapillary array membrane
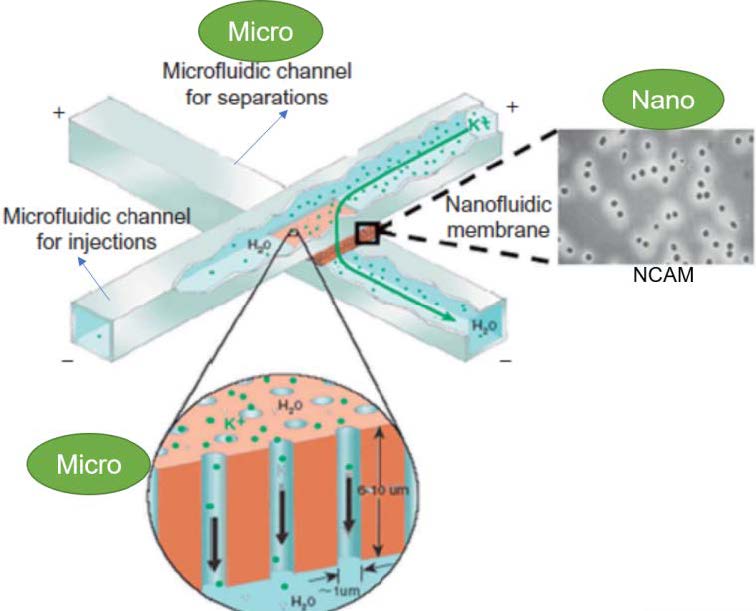
An NCAM (nanocapillary array membrane) sandwiched between two microfluidic channels can be used to electrokinetically inject samples from a source microchannel to a separation microchannel, where electrophoretic separations are performed. Electrokinetics is an electrical process where two electrodes are placed in the ground and a low-intensity direct current applied. Electrophoresis is a powerful technique based on the use of an electric field for the separation of proteins into distinctive patterns.
Review Questions
Circulation in the channel caused by density changes in the fluid due to fluid expansion when heated.
Repeated event of blood vessel narrowing
Formation of a blood clot in the wall of a blood vessel, hindering normal flow of blood
Ion that accompanies an ionic species in order to maintain electric neutrality
Any ion of the same charge as another in a solution or other electrochemical system